Pandemic
Preparedness
The COVID-19 pandemic won't be the last outbreak your hospital will have to face.
Always read the label and follow the directions for use.
Now is the time to update your strategic PPE plan—when you can see, and measure—what your facility needs to be ready.
HIV-AIDS, H1N1 flu, Ebola, and now COVID-19 have all highlighted the critical challenges created by global disease outbreaks, including:
- Lack of public-private sector coordination
- Severe warehousing distribution capacity limitations
- A far-flung global supply chain
- Access constraints caused by airport and port restrictions and border closures.
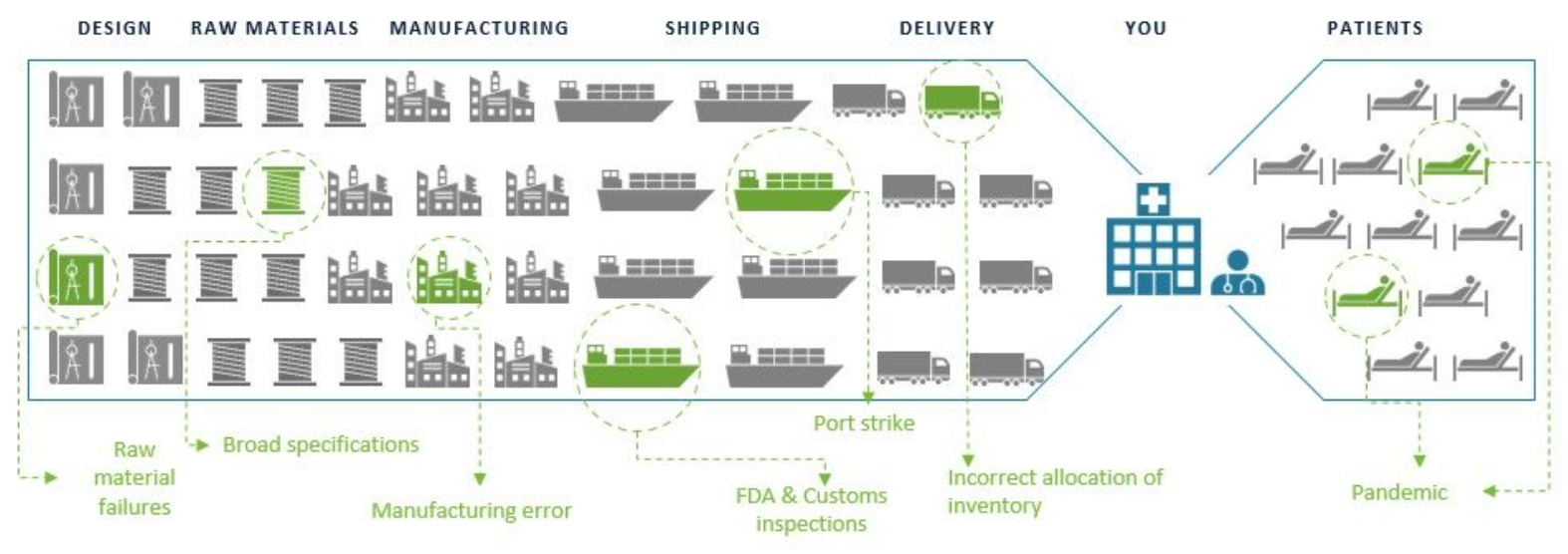
A complex and remote supply chain can break in many places. That’s why implementing a strategic PPE plan matters when it comes to pandemic preparedness.
You can count on HALYARD* to help meet your needs during a pandemic event or infectious disease outbreak.
We invest in proximate production capacity and work with our raw material suppliers and distributor partners to meet current and future increased demand, with limited interruptions to our customers.
10 Questions To Ask Before You Stockpile
10 Questions To Ask Before You Stockpile
The questions posed in this downloadable guide are designed to help you begin creating a stockpiling system that will accomplish your pandemic preparedness goals. Whether the infectious outbreak risk is seasonal flu, pandemic influenza, or a unique pathogen, we can help you determine the type and quantities of PPE needed for your hospital’s unique pathogen scenarios.
NEED HELP ESTIMATING STOCKPILE QUANTITIES?
Contact a HALYARD* representative to help you estimate PPE requirements for pandemic events.
A breakdown at any point in a vendors’ supply chain could directly impact those who need PPE.
From end to end, our vertical integration means that we take ownership of every step in the value chain.
The PPE gear you need for pandemic preparedness
HALYARD* offers quality PPE that meets and often exceeds industry standards for protection from pathogens and fluids to keep healthcare workers safe.
FLUIDSHIELD* Surgical N95 Respirator Mask
HALYARD FLUIDSHIELD* N95 respirators meet the Standards for single-use face masks AS4381, ASTM F2100-11, and EN146834. They are entered in ARTG 351812 as both Medical Respirators and Surgical Masks.
NIOSH approved HALYARD* FLUIDSHIELD* N95 respirators are cleared by FDA as Class 2 medical device to be used in a healthcare setting.
The duckbill breathing chamber is more than twice as large as the leading competitive surgical N95 and exceeds NIOSH standards for breathability.
- NIOSH-Approved
- Splash resistance: 160 mmHg
- BFE: ≥ 99%
- PFE: ≥ 99%
- ASTM Level 3 Protection
- Type: Respirator
- SO SOFT* Lining
FLUIDSHIELD* Surgical N95 Respirator Mask
HALYARD* Isolation Gowns
HALYARD* BASICS* Tri-Layer AAMI2 Over-The-Head Isolation Gown
Provides barrier protection based on AAMI guidelines and helps reduce the risk of contamination and transmission of infectious organisms that lead to HAIs.
- Meets AAMI Level 2 Requirements
- Medium weight 3-layer SMS fabric
- Over-the-head design, tie waist
- Thumb hooks and elastic cuffs
- Ultrasonic Seam Closure
- Closed back
Additional Products to ensure Pandemic safety
Qualitative Fit Test Kits
Request Consultation
Ready to get prepared? Contact one of our representatives today.
