Here’s a question for you. Of the three things listed below, which are Class II medical devices?
- Feeding Tubes
- X-ray Machines
- Surgical Gowns
If you said all of the above, you’re absolutely right! While you might not think of surgical gowns as devices, just as much research and technological development goes into creating personal protective equipment (PPE) as any other tools used in the practice of medicine.
There are many factors to be considered in designing garments that will keep OR personnel and patients safe from cross contamination during surgery. Organizations exist that develop performance standards for different types of apparel. The one that has established the standard recognized as the consensus standard for surgical gowns and drapes by the FDA is the Association for the Advancement of Medical Instrumentation (AAMI).
The “AAMI Standard” (ANSI/AAMI PB70:2012) provides standards for liquid barrier performance in the critical zones of gowns and drapes, the areas where OR personnel are most likely to come in direct contact with potentially infectious material, such as blood and bodily fluids.
What are the five things I should know about choosing a gown?
The first thing you need to know is, none of the AAMI Ratings specify the type of gown by type of procedure. Each facility is ultimately responsible for choosing the right gown for the procedure for its OR staff.
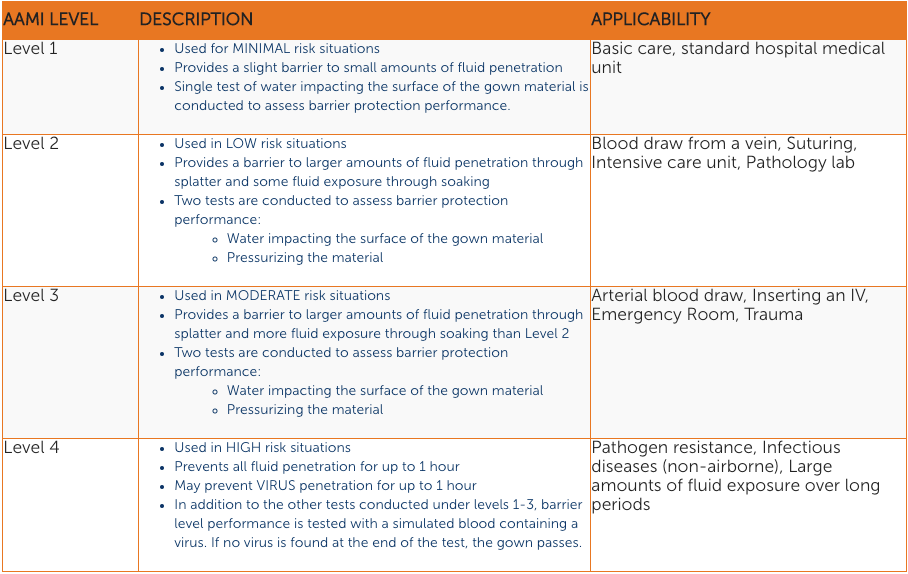
The next four things are the AAMI Ratings for different levels of protection:
- Level 1: Minimal Fluid Barrier Protection – Used for daily patient care, when there is little to no risk of fluid exposure. Generally not used in the OR.
- Level 2: Minimal to Low Fluid Barrier Protection – Used when there’s only a slight risk of fluid exposure, for minimally invasive surgical procedures such as lumps and bumps.
- Level 3: Moderate Fluid Barrier Protection – Used for the widest range of surgical procedures, where the risk of fluid exposure is moderate.
- Level 4: Highest Fluid and Microbial Barrier Protection – Provides protection against bloodborne pathogens in critical zones; used for long, fluid-intensive procedures. (This Level was strengthened by the FDA in 2016 and requires documentation of additional testing [ASTM 1671]2 on the part of gown manufacturers.)
Related Article
Tell me more about the critical zones.
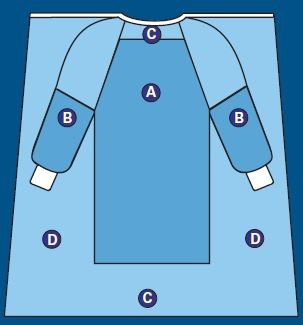
This diagram shows the critical zones on a surgical gown.
Critical Zones are defined as the areas where direct contact with blood, bodily fluids, and other potentially infectious material is most likely to happen (Areas A and B). For surgical gowns, this includes the fabric and the construction (sleeve seams, front tie attachment) in areas A and B.
The entire gown (Areas A, B, and C), including seams but excluding cuff, hems, and bindings, is required to have a barrier performance of at least Level 1.
The back of the surgical gown (Area D) may be non-protective.
AAMI liquid barrier standards also apply to surgical drapes.
Critical Zones on Surgical Drapes
The entire drape is required to be at least Level 1.
The critical zone is usually the center of the drape, around any fenestrations or openings. This area is often reinforced with additional fabric, which may be fluid absorbent.
Any seams between two areas must have at least the barrier performance of the lower performing area.
Summary Of AAMI Rating Levels
Is there anything else I need to know?
If you have any questions about the AAMI Ratings of surgical gowns or drapes provided for your use in the OR, ask your supervisor. Another great resource is the Association of periOperative Registered Nurses (AORN). The AORN Guidelines for Perioperative Practice offer comprehensive guidance to the types of gown protection needed for operative and other invasive procedures.
For a handy reference, download our Simplified Guide to Gown Guidelines poster.
Sources
- https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns#g5
- ASTM F1671 / F1671M-13, Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System, ASTM International, West Conshohocken, PA, 2013, http://www.astm.org/cgi-bin/resolver.cgi?F1671F1671M
- https://nsf-muses.ucdavis.edu/sites/g/files/dgvnsk1751/files/files/persentations/Barrier-Performance-According-to-AAMI-PB70-Peter-Brown.pdf, Page 5
